Calcium-free Recoverin

PDB ID: 1IKU
Medline Link
PDB file
Calcium-bound Recoverin
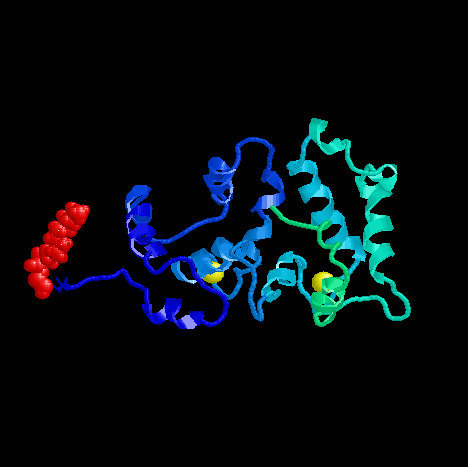
PDB ID: 1JSA
Medline Link
PDB file
Biology
Calcium-myristoyl switch
The most prominent feature of recoverin is the calcium-myristoyl switch. Myristoryl group is normally sequestered away in the protein's hydrophobic interior binding pocket. When the protein binds calcium ions, change in conformation brings out myristoyl group from the binding pocket so that the myristoyl group can interact with the target or the protein can translocate to a different region.This type of calcium-myristoyl (or other acyl group) switch arose early
in evolution of eukaryotes. The key residues that allow the conformation
change is conserved in many such proteins from yeast to humans. Also, the
key residues that make up the binding pocket of myristoyl group in the
calcium-free state is also invariant.
Rhodopsin, Recoverin and Vision
Rhodopsin is a light receptor molecule. When a photon strikes rhodopsin, rhodopsin changes its conformation, and a series of molecular signaling events take place. The end result of all the molecular event is the sending of a neural signal to the brain to report that a photon has been received.Eyes of mammals have various ways of adapting to many different light levels. One is by adjusting the size of the iris. The other is by tagging phosphate groups to rhodopsin so that rhodopsin is less likely to initiate the signaling cascade. Phosphates are attached by rhodopsin kinase (RK).
Recoverin is a calcium-binding protein with a relative molecular weight of 23 K. Recoverin is involved in the recovery phase of visual excitation and in adaptation to the background light. Calcium-bound form of recoverin slows down the activity of RK, thereby prolonging the light sensitivity of rhodopsin.
Interesting to note is the location of recoverin molecules. When recoverin
is not bound to calcium, it stays in cytosol. This is probably because
the myristoyl group is hidden inside the protein. When recoverin binds
calcium, it migrates to the disc membrane and embeds itself into the membrane
using the myristoyl group as the anchor. Myristoyl group is attached to the N-terminal glycine. It is in an extended
conformation. It is embedded deep into the protein, and is stabilized by
five helices that provide hydrophobic and aromatic residues for the binding
pocket. Helices B, C, E and F provide walls of the pocket, while helix
A provide a "lid" for the binding pocket.
This change is brought on by three key changes in conformation when
calcium binds EF-2 and EF-3. C-terminal domain does not change much when
calcium binds. First, the orientation of the helices in the EF-1 changes
to the open conformation (helices become perpendicular to each other) without
binding calcium, much like classical calcium-bound EF-hand motif. Second,
changes in the conformation of EF-2 and EF-3 when bound to calcium ion
brings about the rotation at Gly96. This rotation results in the two domains
rotating 45 degrees relative to each other when compared to calcium-free
recoverin. Third, the binding of calcium brings about a rotation at Gly42.
All these changes lead to two important events. One, the hydrophobic
pocket that provided a resting place for the myristoyl group is no longer
present. Thus, the myristoyl group is free to move out. Two, the N-terminal
end of recoverin swings itself out of the binding pocket. This results
in physically pulling on myristoyl group, and subsequent ejection of the
acyl group.
All these remarkable changes lead to the activation of recoverin. Myristoyl
group can now look for its target in the membranes. Also, the exposed hydrophobic
residues can now interact with their targets as well.
Structure of Calcium-free Recoverin
Recoverin is composed of 11 alpha helices (named A to K) and 2 pairs of
short beta sheets. Four pairs of the helices form EF-hand-like helix-loop-helix
motifs. Overall, the EF-hand-like motifs are linked in a linear fashion,
as compared to the two-domain dumbbell shape of calmodulin,
which also has four EF-hand motifs.
Structure of Calcium-bound Recoverin
The first notable change is the location of myristoyl group. Compared to
the tightly tucked-in conformation of myristoyl group in the calcium-free
state, calcium-bound state shows a remarkable change in conformation that
brings out the myristoyl group to the exterior of the protein.
Refences
Ames
JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Molecular mechanics
of calcium-myristoyl switches. Nature 389, 198-202 (1997).