|
Classical
cadherin molecules possess functional sites for adhesive
recognition, calcium binding, membrane integration,
cytoskeletal interactions and posttranslational modifications
such as glycosylation, phosphorylation and proteolysis.
All classic cadherins are contain a conserved cytoplasmic
domain in the carboxyl terminus that interacts with
the catenins characterized by Armadillo-repeats. The
cytoplasmic domain has a serine-rich region that interacts
with b-catenin. b-catenin then binds to a-catenin which
connects the cadherin-catenin complex to the actin cytoskeleton.
More recently, it was found that a different group of
catenins binds to cytoplasmic domain including include
p120 and d-catenin.
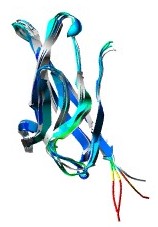
NMR
structure of Ncad1
In
1995, Overduin determined the structure of the first
domain of E-cadherin using NMR spectroscopy. Subsequently,
the crystal structure of the first domain of N-cadherin
(Ncad1) was solved. The fold consists of a seven-strand
b-sheet (A, A', B,C,D,E,F,and G. A and A' parts are
parts of the same strand), with the N and C termini
located at opposite ends of the molecule. The segment
connecting strands B and C adopts an apparently helical
structure made of a succession of b-turn and b-like
hydrogen bonds. This unique quasi-b-helix structure
is characteristic of the cadherin fold.

Ecad12
Homodimer
The
crystal structure of the E-cadherin fragment containing
domains 1 and 2 (Ecad12) showed that calcium is central
in E-cadherin dimer formation. Within the dimer, there
are three Ca2+ ions bound per cadherin molecule. The
residues that are involved in binding of three Ca2+
ions are located around the linker region between domain
1 and 2: Glu11, Glu69, Asp100, Gln101, Asn102, Asp103,
Asp136, and Asn143. These Ca2+ binding sites were also
found in the crystal structure of the N-cadherin fragment
containing the two N terminus cadherin repeats (Ncad12).
Single amino acid substitutions in the calcium binding
sites can disrupt cell aggregation in vivo . Early biochemical
and biophysical data suggested several explanations
for this dependence, including rigidifying cadherin
structure and conferring resistance to proteolysis.
Crystal
structures of both Ecad12 and Ncad1 show parallel homodimer
formation, however the dimer interfaces are different.
The homodimer of Ncad1 does not depend on Ca2+ whereas
Ecad12 requires Ca2+. In the crystal structure of the
Ncad1, the Trp 2 side chain is intercalated into the
hydrophobic core of the partner forming an intimate
'strand' dimer. This type of interface was not observed
in a subseqeuent crystal structure of Ncad12. On the
other hand, Ecad12 forms a weak homodimer mediated by
calcium ions and water molecules with contacts that
are mostly located in the linker region between the
domains.

Possible
lattice structure
Structural
and biophysical studies on E-cadherin support a model
in which parallel dimerization of cadherin molecules
are mediated by Ca2+ ions. In this model, parallel dimerization
is prerequisite to cell adhesion. Prior to calcium binding,
the cadherin monomers are flexible at the linker region,
so they can have many conformations. Upon calcium binding
at the linker region, the cadherin molecule rigidifies,
adopting a conformation where cadherins can dimerize
loosely, as exemplified by the weakness of the Ecad12
dimer. The stability of a weak dimer would depend on
the dimerization of adjacent cadherin molecules in a
lattice structure. The lattice would form only if a
critical concentration of cadherins on the cell surface
is met. The dimerization step coupled with perhaps interactions
in the cytoplasm or other cadherin repeats are required
for the formation of an ordered lattice of cadherins.
Finally, the cadherin lattice adheres in a calcium independent
manner with another cadherin lattice formed at the opposite
cell surface to achieve cadherin-mediated cell-cell
adhesion.
Although
an early study on electron microscopy imaging of extracellular
cadherin constructs suggested that interactions between
cadherin monomers are limited to the N-terminal tip
of the molecule, there is increasing evidence that other
extracellular parts of cadherins and/or the intracellular
domain are involved in lateral clustering and ultimately
cell adhesion. Using atom force microscopy, Sivasankar
demonstrated that the extracellular domain of cadherin
exhibits multiple adhesive contacts possibly involving
other repeats in the molecule. In addition, the dimensions
of the cadherin molecule suggest the likely involvement
of other cadherin repeats in cell-cell adhesion. A single
cadherin repeat spans ~45 angstroms and upon rigidification
by calcium binding, the complete extracellular domain
of cadherin spans ~240 angstroms. If the adhesion interface
involves only the N terminal cadherin repeat, then the
distance between opposite cells should be ~440 angstrom,
however, the distance between the cell-cell distance
is ~250-350 angstroms. Therefore, to conform to that
constraint, there may be lateral interactions involving
other cadherin repeats.
Thus,
future structural work will be focused on elucidating
the entire extracellular domain, as well as the more
elusive cytoplasmic domain interacting with b-catenins.
The structure of the b-catenin armadillo repeat reported
by Huber and recently the structure of a-catenin reported
by Pokutta will ultimately serve, in conjunction with
the information available for cadherins, as a basis
for understanding of cell-cell adhesion and various
signaling and cell development events.
|