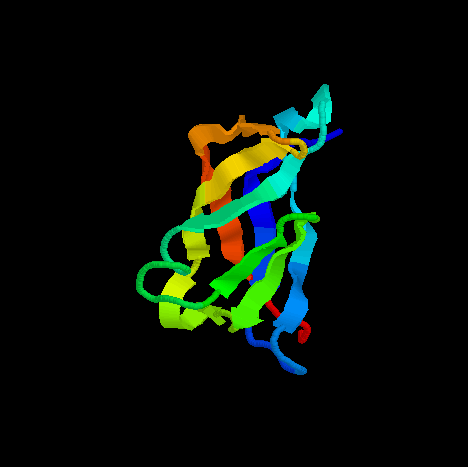
PDB ID: 1SUH
Medline link
PDB file
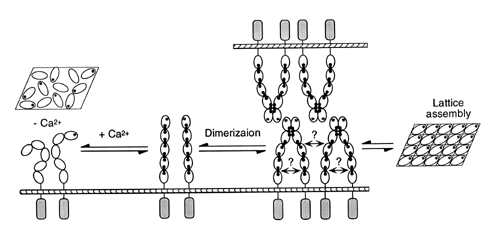
Possible mode of E-Cad interaction.
Taken from Alattia et al, 1997.
Biology
Molecules that mediate cell-cell interaction or adhesion are very important in development and maintenance of multicellular organism. The moment a fertilized cell divides, there must be a way to hold these cells together in order to form a single entity. As various organs and tissues develop, there must be a way to distinguish one another. Cell-adhesion molecules provide not only a glue to hold them together, but also a way of distinguishing one tissue from another.Cadherins are a family of cell-surface molecules that provide calcium-dependent cell-cell adhesion. Each tissue expresses a different cadherin, resulting in tissue formation. Epithelical cells express epithelial cadherins (E-Cadherin) that provide epithelial surface integrity. Without E-cadherins, tumor cells will have much easier time invading into other tissues. Immunoglobulin cell adhesion molecules (ICAMs) on the other hand provide calcium-independent cell-cell adhesion.
Cadherins typically contain five extracellular repeats (CAD's), a transmembrane
region, and a cytosolic region. N-terminal domain (CAD1) repeat is the
one that is able to recognize "like" cadherins and bind them together.
Structure
E-CAD1 contains seven beta sheets and two very short alpha helices. Most of the beta sheets are anti-parallel to each other except one pair (A' and G are parallel to each other). Overall, seven anti-parallel beta sheets form a beta-barrel topology.The structure contains two important areas: metal-binding pocket, and homophilic specificity-conferring surface. The two regions are 20 A apart from each other. However, without calcium in the metal-binding pocket, the homophilic specificity-conferring surface does not work.
Metal-binding pocket is composed of DAD sequence, where the glutamate
residues provide the negative charge for binding positively charged calcium
ions. Homophilic specificity-conferring surface is made up of HAV motif
starting at residue number 79. Additional residues seem to participate
in the homophilic specificity as well. At first, the overall topology may
be similar to the immunoglobulin superfamily (Ig superfamily). However,
there are enough differences to keep them as a separate family. First,
the disulfide bond commonly found in Ig family is not found in E-cad. Second,
E-CAD1 forms many hydrogen bonds between beta sheets to form a beta barrel,
where as Ig family does not. Third, the metal binding pocket of E-CAD1
is not present in Ig family.